Park NX10 SICM Module
The new standard for nanoscale imaging in aqueous environments
Park NX10 SICM provides
nanoscale imaging for a wide range of applications:
-
Cell Biology
Cell morphology imaging, nano biopsy and injection
-
Analytical Chemistry
Electrochemical reaction imaging by integration of scanning electrochemical microscopy
-
Electrophysiology
Ion channel detection together with patch clamping
-
Neuroscience
High resolution imaging of single neuron integrated with patch clamping
Brochures
Park SICM Technology
Incredibly Non-invasive and Easy-to-use In-liquid Imaging
Truly non-invasive in-liquid imaging with SICM
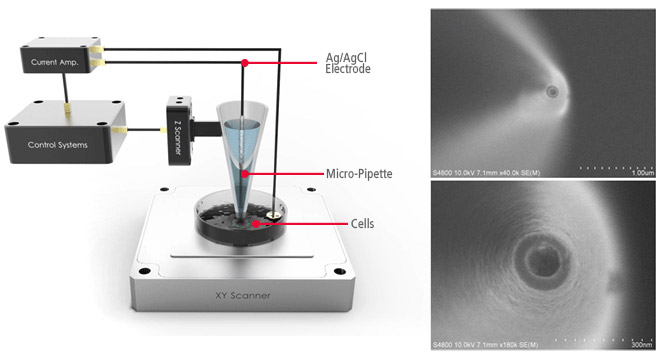
Park SICM uses nanopipettes
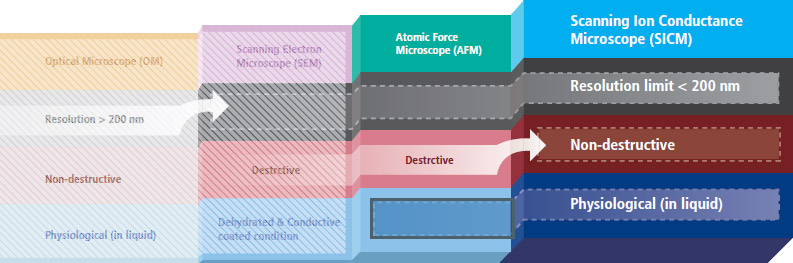
In Scanning Ion Conductance Microscopy developed by Park Systems (Park SICM), a glass nanopipette filled with an electrolyte acts as an ion sensor that provides feedback on its location relative to a sample completely immersed in liquid. The pipette tip maintains its distance from the sample by keeping the ionic current constant. In comparison, AFM typically relies on interaction of forces between its probe tip and the sample. The pipette has an inner diameter about 100 nanometers, made of glass.
No Force, Non-Contact imaging in Liquid
Similar to Scanning Tunneling Microscopy (STM) operating in ambient air, the Park SICM operates in liquid without making physical contact with the sample. Electrodes on either side of the sample and pipette produce ionic current that flows through the surrounding solution. A sensor measures the current flow, which decreases as the distance between the pipette and sample becomes smaller, and monitors the distance between the pipette and the sample to obtain the topology.
Our dedicated auto-imaging software makes scanning easier and more accurate
Automation for easier scanning
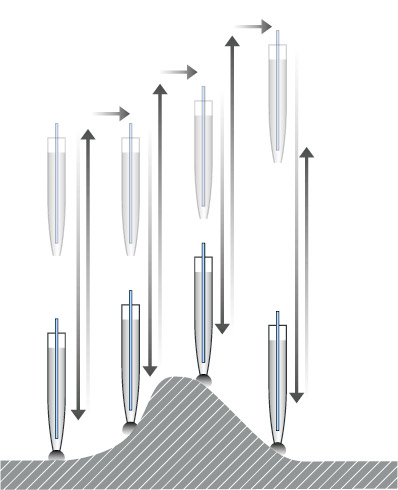
Streamline research and increase productivity with ARS (approach- retractscan) free from parameter controls, so you have less to worry about while scanning.
Steady pipette probe-sample distance control in nanoscale
By automatically refreshing its reference value before approaching each pixel, the stopping height of the pipette near the sample surface is not influenced by set-point drift.
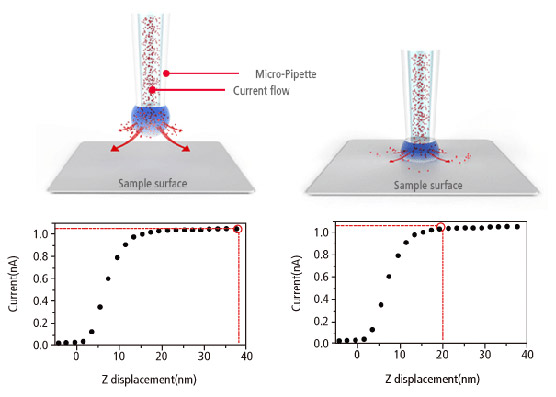
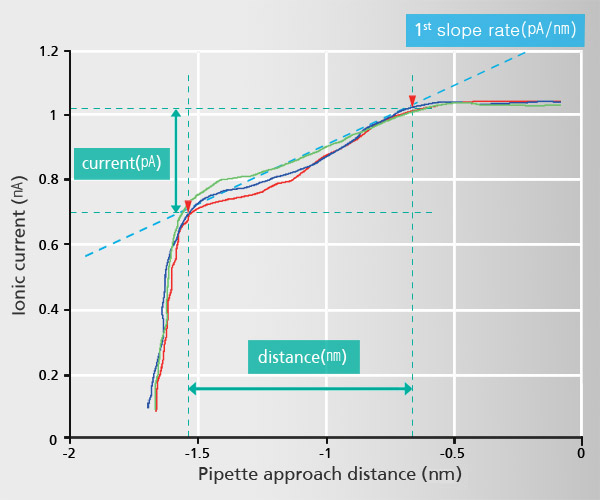
Current-Distance(I-D) Spectroscopy
Acquiring Current-Distance Curve of SICM on the way of pipette approach (vertical direction movement) toward sample surface is beneficial to elucidate various biological and chemical phenomena in aqueous environment.
This beneficial application can be applied to a specific and interesting object of sample, identified with SICM’s non-invasive topography. Furthermore, utilizing ‘current-distance curve mapping’ function enables researcher examine and acquire the current-distance curve at multiple positions so that they can reach to deeper level understanding for biological and chemical reaction research.
Simple setup and operation
The SICM head can be easily added to Park NX10 platform by sliding it into a dovetail rail. This auto-engages a head connection that allows users to fully control device electronics, making setup and operation fast and simple. With the addition of two electrodes connecting to the pipette and sample buffer, researchers can generate and acquire ionic current signal through the pipette’s openings. Furthermore, a vertically aligned motorized z stage allows users to easily adjust the pipette’s height positioning.
Park NX10 SICM Module
The integration of the SICM and Park NX10 AFM system from Park Systems allows researchers to expand the depth of their research and easily perform nanoscale imaging in aqueous environments.
High speed Z-scanner with 15 μm scan range
Driven by a high-force piezoelectric stack and guided by a flexure structure, the standard Z-scanner has a high resonant frequency of more than 9 kHz.
Low noise Z position sensors
The industry leading low noise Z detector enables Park SICM to capture nanometer size feature of sample providing nano-scale precision of sample topography recording. This produces highly accurate sample topography, no edge overshoot and no need for calibration.
Low ionic current noise level of Park internal current amplifier
The internal current amplifier of the Park SICM provides the optimized current signal processing environment for accurately recording pico-ampere current signal of SICM feedback.

Intuitive Pipette Holder of SICM head with Exchange Kit
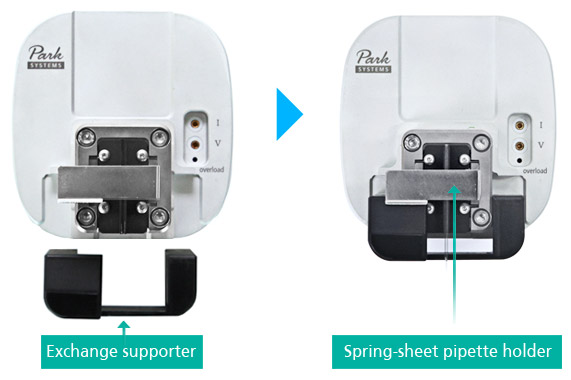
With our user interface viewpoint, Park NX10 SICM head has user-friendly and intuitive holder design. It is easy and simple to install and exchange pipette on Park SICM head without additional pipette holder. By inserting ‘exchange supporter’ to SICM head, it lifts up ‘spring-sheet pipette holder’ so that nano/micro-pipette can be inserted to holer in easy without pipette damage. This new design of pipette holding also led more clear in optical view and faster SICM imaging speed.
Faraday cage for stable SICM operation

Designed exclusively for Park NX10 SICM platform, the faraday cage effectively protects the pipette, head, and XY scanner from interference, providing a more stable scan environment. The transparent conductive mesh blocks electric fields and shields external static or non-static electromagnetic field of 50/60 Hz, while still allowing researchers to maintain a clear view of the pipette and sample.
Specifications
SICM Head with
pipette probe holder
- Includes a low-noise, high-precision ionic current amplifier
- Includes a high-force Z-scanner
- Flexure-guided structure driven by multiply-stacked piezoelectric stacks
- Z-scanner range: 15 µm
- 20-bit Z position control and 24-bit Z position sensor
- Dovetail lock head mount for easy mount/removal of the SICM head
- Automatically connects to the electronics upon mounting
Supported Modes
- SICM Standard Imaging
- DC (direct current) mode, ARS (apporach-retract-scan) mode
- SICM Ionic Current Measurement
Application Options
Live Cell Study with Live Cell Chamber (SICM & AFM)
The live cell chamber creates an ideal environment for cells, improving their life expectancy during long measurement durations through controlled temperature, pH, and humidity at optimal conditions. Experiments with the live cell chamber have demonstrated cell survivability of more than 20 hours.
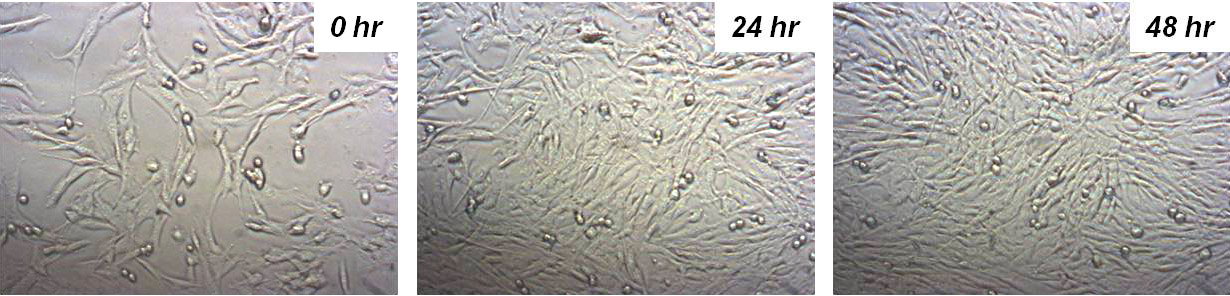
Human fibroblast cells in the Live Cell Chamber of NX-Bio survive over 48 hours.

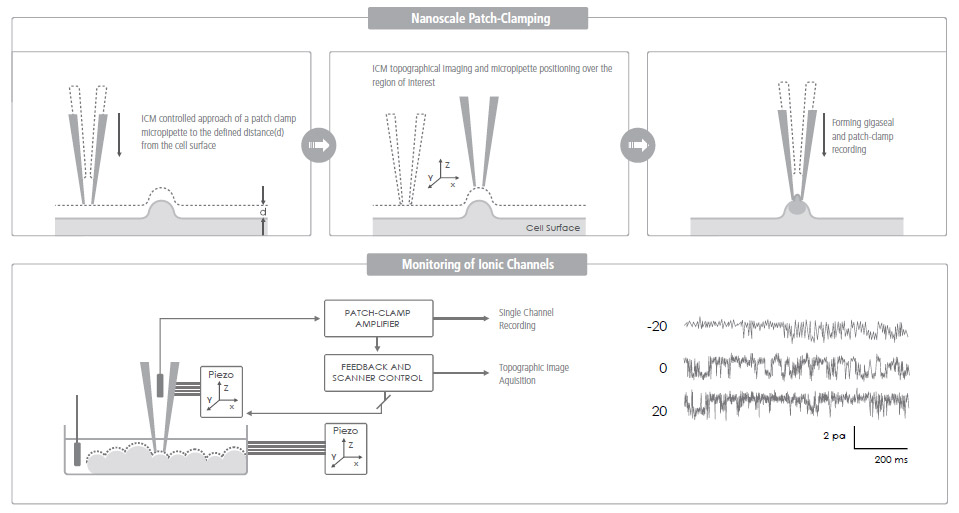
Ion Channel Recording of Targeted Patch Clamping
Conventional Patch Clamping is an optical microscope view-based technique used to monitor a single living cell's ion channel activity—a key quantifier of various cellular activities. Targeted Patch Clamping is the SICM-based version of this technique that enables the detection of ion channel activities of specific subcellular structures.
SICM + Patch Clamping = Targeted Patch Clamping
More Comprehensive Cell Biology Study, by Integrating Fluorescence Microcopy with Park SICM
Combining fluorescence microscopy (FM) techniques with Park SICM can create new benefits and provide comprehensive information for cell biology studies that cannot be obtained when using only one of those techniques. While monitoring external cellular surface morphology with SICM, the internal cellular behavior can be observed by FM.
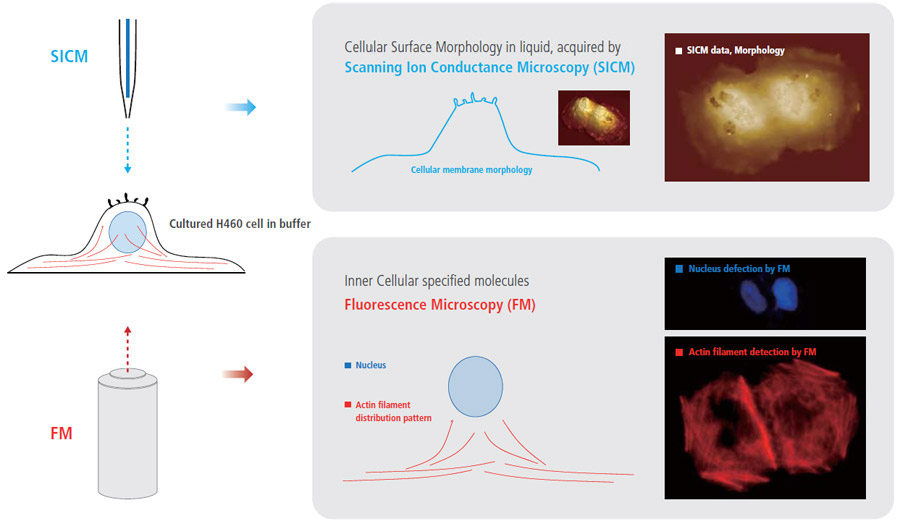
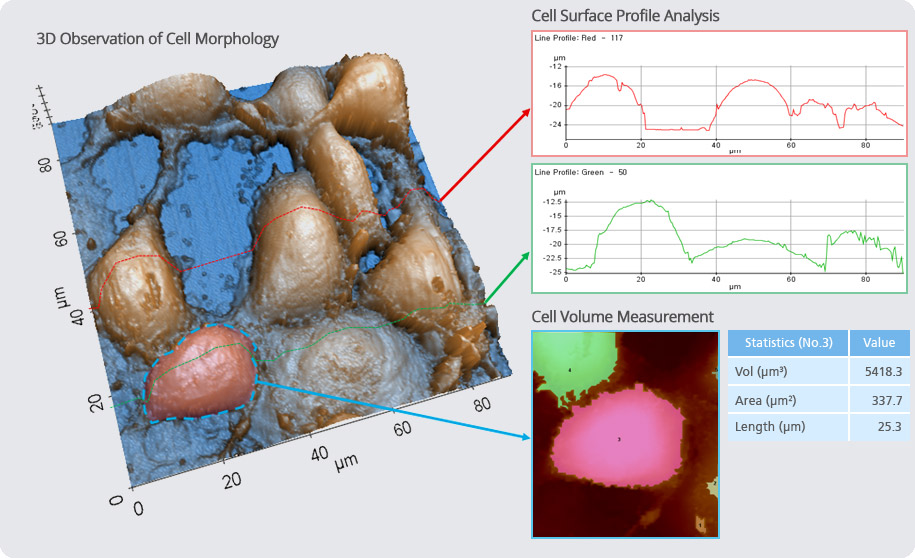
Structural & Physical Property Characterization of Cell in XEI
Software Option
SmartScanTM – Data Acquisition
SmartScanTM is a data acquisition software that provides all user controls of Park AFM measurements. The friendly user-oriented interface of SmartScanTM provides easy operation of the AFM.
• Simultaneous data acquisition of up to 16 images
• Maximum 4096 × 4096 pixels image size
• Dedicated Force-distance and I-V spectroscopy with batch processing
• Cantilever spring constant calibration
XEI – Image Processing and Analysis
XEI is the AFM image processing and analysis program. Its powerful processing algorithms make analyses easy and streamlined. With its most advanced and versatile imaging features, AFM users can obtain essential and critical information from their experiment.
• Image analysis of line profile, region, 3D rendering
• Spectroscopy data analysis module (F-d, I-V)
• Directly copy/paste to presentation program
• Multiple image comparison
• Image overlay of two different images
Image Overlay: SICM Topography + Fluorescence Microscopic Image
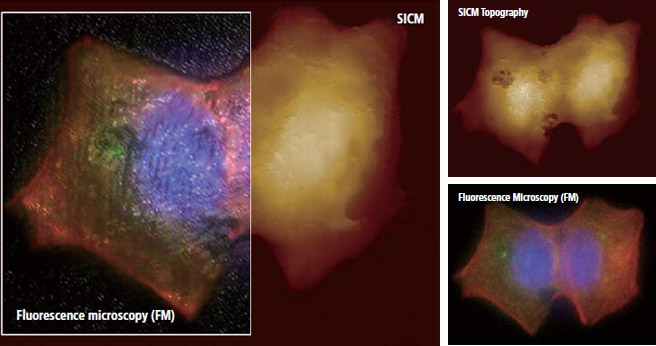
Park Systems’ Image overlay software allows to combine fluorescence microscopic image onto SICM topography accurately. This dedicated software helps a complicate combining process much easier .
- SICM Technology
- AFM Technology
- SICM + AFM
The SICM of Park NX12 is the next generation nanoscale microscope for life science
Park SICM can acquire biological images at nanoscale in physiological conditions, attaining high resolution of less than 200 nm. The biological images obtained from SICM are free from morphological deformation, which can occur from scanning electron microscopy (SEM) or even AFM systems.
Park SICM uses nanopipettes
In Scanning Ion Conductance Microscopy developed by Park Systems (Park SICM), a glass nanopipette filled with an electrolyte acts as an ion sensor that provides feedback on its location relative to a sample completely immersed in liquid. The pipette tip maintains its distance from the sample by keeping the ionic current constant. In comparison, AFM typically relies on interaction of forces between its probe tip and the sample.

AFM uses a micro-thin cantilever and tip as a probe. For Park SICM, the pipette is a probe with an inner diameter ranging from 80 to 100 nanometers for pipettes made of glass and 30-50 nanometers for those made of quartz.
No Force, Non-Contact Imaging in Liquid

Similar to Scanning Tunneling Microscopy (STM) operating in ambient air, the Park SICM operates in liquid without making physical contact with the sample. Electrodes on either side of the sample and pipette produce ionic current that flows through the surrounding solution. A sensor measures the current flow, which decreases as the distance between the pipette and sample becomes smaller, and monitors the distance between the pipette and the sample to obtain the topology.
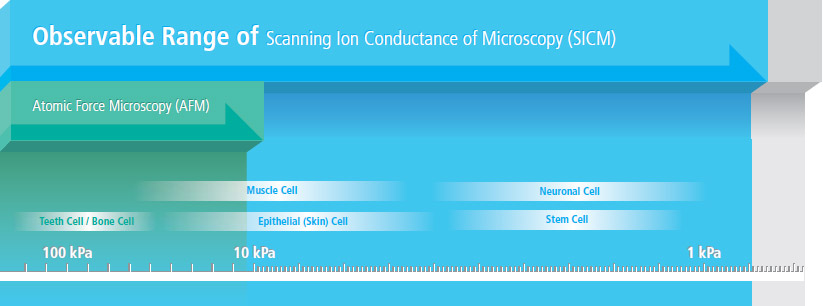
Park SICM Can Image All Cell Types
- Park SICM can image even the softest cells such as the neuron cells, live—something that’s impossible with any other microscopy techniques.
- SICM can even image suspended network of neurons
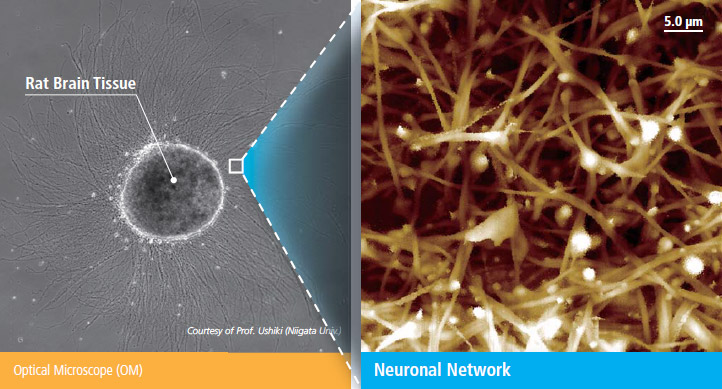 Courtesy of Prof. Ushiki (Niigata Univ., Japan)
Courtesy of Prof. Ushiki (Niigata Univ., Japan)
Advanced Park AFM Technology Enables Accurate Force-distance Spectroscopy
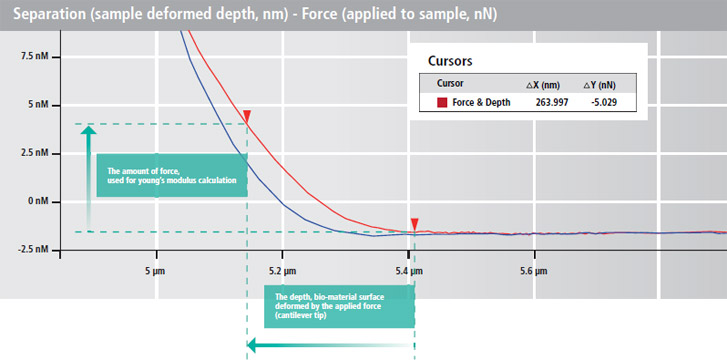
Force-distance (FD) spectroscopy using an AFM is a beneficial tool to characterize bio-mechanical properties of various biological materials. In FD spectroscopy, the cantilever tip touches the sample surface with a user prescribed amount of force accurately applied using the AFM’s Z scanner. Park AFM’s industry leading low noise Z detector allows the researcher to control Z scanner movement to apply an exact amount of force very accurately to a sample surface during the FD spectroscopy. This enables the researcher to collect detailed bio-mechanical characterization data at the nano-newton scale.
- Nanomechanics of Single Muscle Fibers by AFM
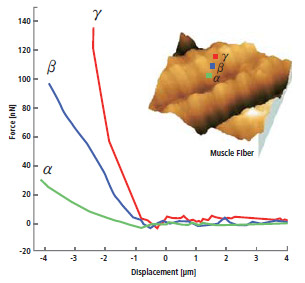 International Nano-Conference(ICN+T), Basel (CH), 2006, Noemi Rozlosnik Technical University of Denmark
International Nano-Conference(ICN+T), Basel (CH), 2006, Noemi Rozlosnik Technical University of Denmark
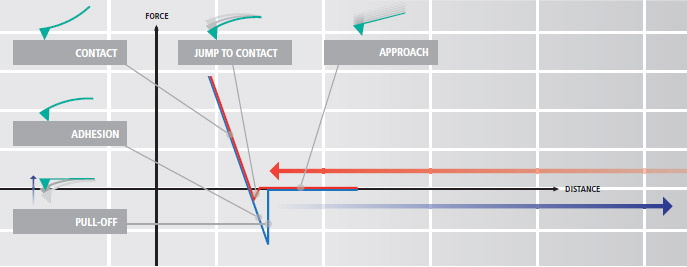
- Force Distance Spectroscopy measures the mechanical interaction force between the tip end and the sample.
- The force-distance curve is acquired by indenting the cantilever to sample surface.
Advanced Bio-mechanical Property Measurement by Calculating Elastic Modulus (Young’s Modulus)
The Herzian and Oliver Pharr models are calculated automatically from the Park AFM's accurate FD spectroscopy data to determine the elastic modulus (Young's modulus). Both of these calculation methods are included in Park XEI, the data analysis software in Park NX12. They strengthen the biomechanical data verification of FD curves obtained in your experiments.
- Acquiring the actual depth, sample deformed by applied force
(separation - force curve)
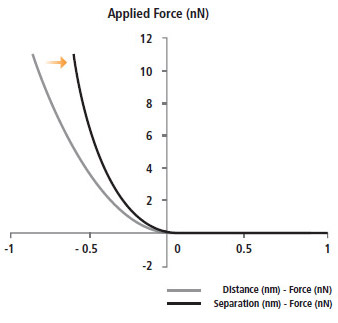
- Calculating Young's Modulus in Hertzian model
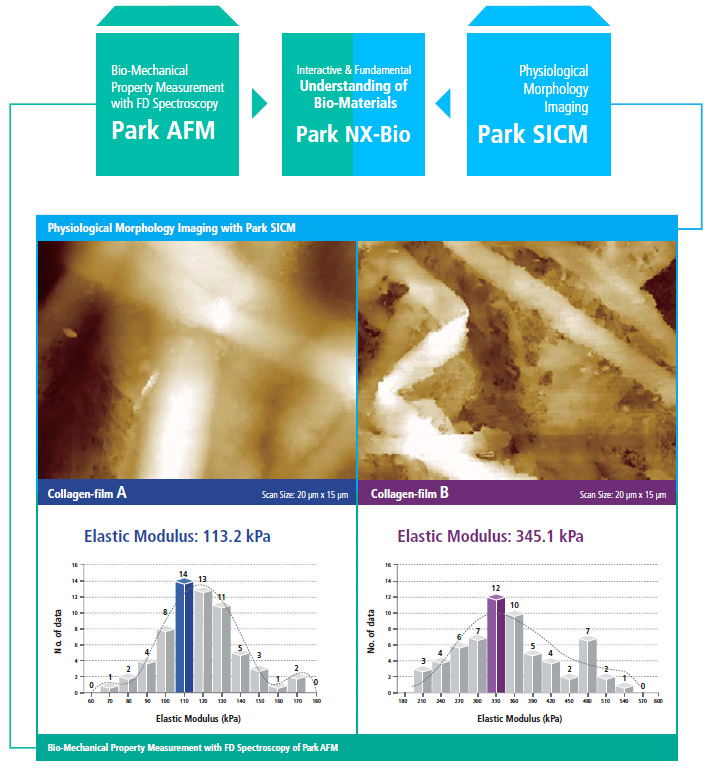
Park SICM and Park AFM Technologies Put Together
Outstanding Investigation Tool for Biological Research by Combining Physiological Morphology with Bio-Mechanical Property Measurements
Park NX12 combines Park SICM’s ability to interpret morphology under true physiological conditions and Park AFM’s capacity to acquire bio-mechanical property data (elastic modulus) accurately. This enables researchers to understand the fundamentals of their biological materials at a deeper level.
Specifications
Scanner
XY scanner range: 100 μm × 100 μm
AFM head Z scanner range: 15 μm, (optional 30 μm)
SICM head Z scanner range: 15 μm, (optional 30 μm)
Electronics
ADC: 18 channels
24-bit ADCs for X, Y, and Z scanner position sensor
DAC: 17 channels
20-bit DACs for X, Y, and Z scanner positioning
3 channels of integrated lock-in amplifier
Spring constant calibration (Thermal vibration method)
Digital Q control
AFM/SPM Modes
Basic modes: True Non-contact™ mode, Tapping mode, and Phase imaging, Contact mode and LFM, PinPoint™ imaging, F/D spectroscopy, Force volume imaging, MFM, Enhanced EFM (Basic EFM, DC-EFM, PFM and SKPM), FMM, Nanoindentation
NX option modes: C-AFM Options (Basic C-AFM, ULCA, VECA, SSRM), High Voltage option, SCM, SThM, STM
Vision (AFM)
Direct on-axis vision of sample surface and cantilever
Field-of-view: 840 μm × 630 μm (with 10× objective lens)
Camera: 5 M Pixel (default), 1 M Pixel (optional)
Objective lens
10x (N. A. 0.21) ultra-long working distance lens
20x (N. A. 0.42) high-resolution, long working distance lens
Software - Park SmartScan™
• AFM system control and data acquisition software
• Auto mode for quick setup and easy imaging
• Manual mode for advanced use and finer scan control
Software - XEI
• AFM data analysis software
• Stand-alone design—can install and analyze data away from AFM
• Capable of producing 3D renders of acquired data
Inverted Optical Microscopy
Objective lens: up to 100x
Fluorescence microscopy* (optional)
Confocal microscopy* (optional)
GloveBox (Optional)
• Allows precise control over the humidity
• Makeup of specified gaseous environments
• Allowing you to insulate highly reactive materials
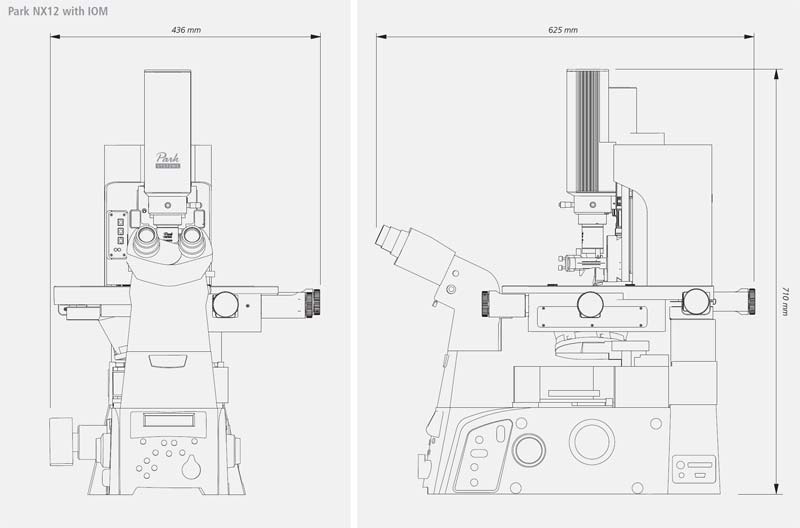
Dimensions in mm