Investigation of lipid bilayer formation by AFM
Introduction
Cell membranes are highly complex structures in which a variety of cellular activities occur in response to both specific and non-specific extracellular signals [1,2]. Extracellular signals transmitted by membrane receptors regulate gene expression and regulate cell proliferation. Although the lipid bilayer constitutes the major structural component of the cell membrane, extracellular matrix (ECM) proteins coat the lipid membrane and provide a reactive surface for other cells and biomaterials to come into contact with [3,4,5]. Therefore, understanding the cell membrane is the first step in the study of cells, the fundamental building block of life itself. In this app note, we present a fundamental study on the lipid bilayer, which is the basic structural component of a cell membrane. As a first step, a defect-free lipid bilayer formation is essential. To form a continuous film, lipids are formed into vesicles using the extrusion method. These vesicles adsorb onto the solid support, where neighboring vesicles will coalesce. When a critical size is reached, the adsorbed vesicles rupture upon forming discs of bilayers that will eventually merge into a film. This bilayer formation strongly depends on the interaction between two vesicles and between a vesicle and the solid support, which is commonly silicon dioxide due to its hydrophilicity. (Fig.1). To investigate the lipid bilayer, a few measurement techniques are adopted such as the quartz crystal microbalance (QCM), which is a nanogram sensitive weighing balance that utilizes the piezoelectric properties of quartz crystal as the working principle [6], fluorescent recovery after photobleaching (FRAP), which utilizes the recovery of fluorescence intensity in an area of the membrane where the fluorophores are bleached [7]. The atomic force microscopy (AFM) [8] is a good candidate for investigating the lipid bilayer. It has the advantage of being able to measure the surface of the sample on a nanometer scale as well as in liquid conditions. In addition, it is an excellent technique for observing the lipid bilayer from various viewpoints because it can measure not only the image of the surface but also the physical and mechanical properties of the sample.
In this app note, a fundamental study of the lipid bilayer formation is conducted using AFM. The formation of the lipid bilayer was confirmed using Non-contact and Contact mode and mechanical properties of the lipid bilayer are investigated by force distance spectroscopy by AFM.
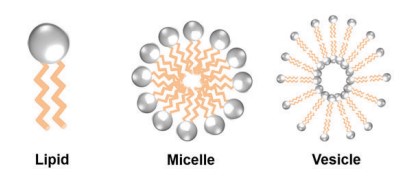
Fig. 1. Structure of lipid, micelle and, vesicle
Materials and Methods
The extrusion method was used to prepare vesicles consisting of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). To create a vesicle with a 50±5 nm diameter, the precursor solution is passing through 100-nm, followed by 30-nm size of filter membranes several times using an extruder. This size-controlled vesicle solution was used to prepare intact vesicle and lipid bilayers on SiO2 substrates [9]. The Contact and Non-contact mode was applied for vesicles or lipid bilayer surface images and force distance spectroscopy was conducted for measuring physical information of lipid bilayer formation. A sharp, silicon nitride AFM probe (BL-AC40TS, 110 kHz resonance frequency, and a 0.09 N/m spring constant) was used for Non-contact mode imaging and force distance spectroscopy and relatively hard AFM probe (BL-AC160TS, 300 kHz resonance frequency, and a 26 N/m spring constant) was used for scratching the lipid bilayer formation in Contact mode. All AFM measurements were performed in liquid (10 mM Tris buffer solution [pH 7.5] with 150 mM NaCl).
Lipid bilayer images by AFM
As shown in Fig. 2, vesicle adsorption on the SiO2 substrate and lipid bilayer formation by ruptured vesicle were imaged on the nanoscale. The SiO2 substrate shows a very flat and uniform surface (Rpv (peak to valley): 2.38 nm) from which the rougher vesicles are easily distinguished, confirming the expected diameter of 50 to 60 nm. Although the lipid bilayer could also be distinguished from the substrate by an increased surface, direct thickness measurement and the detailed shape of the lipid heads (hydrophilic top part of the lipid molecules) were not accessible due to its very flexible and soft structure. Nevertheless, we confirmed the bilayer formation and measure its thickness by intentionally scraping an area with a silicon tip to remove the bilayer locally. For that, the surface of the lipid bilayer was imaged in Non-contact mode, and then a smaller area within the previously imaged area was “imaged” in Contact mode with high loading force. After that, the larger area was imaged again in Non-contact mode and compared with the first image. As can be seen in Fig. 3, in the area scraped in contact mode, the lipid bilayer was locally removed, exposing the bare substrate. The line profile of this hole reveals a depth of about 6 nm, which matches the thickness of the lipid bilayer very well.
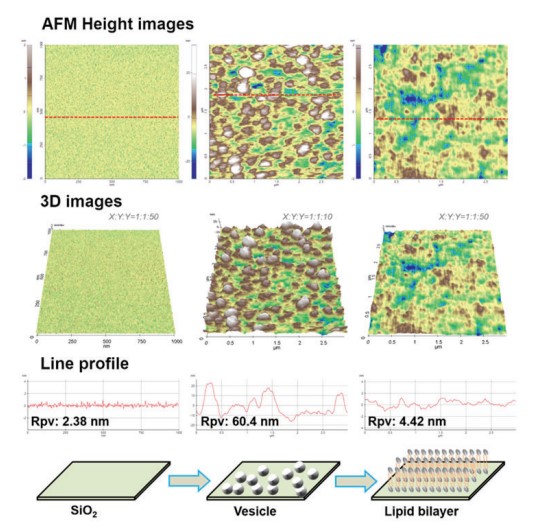
Figure 2. AFM images of the solid substrate (SiO2),
vesicles, and lipid bilayer together with 3D images and line profiles.
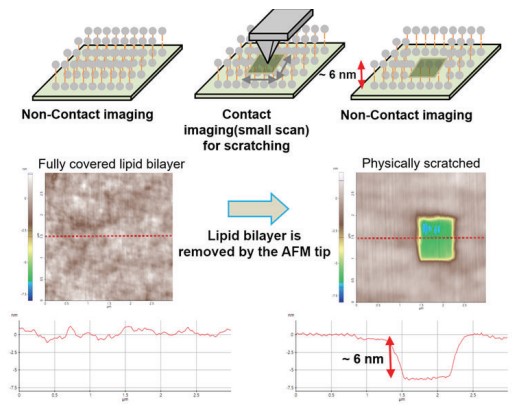
Figure 3. Confirming the lipid bilayer formation by physically scratching.
Physical information of lipid bilayer by force distance spectroscopy
Moreover, we confirmed the bilayer formation using force distance spectroscopy. By measuring the nanomechanical properties locally, it is possible to distinguish if a specific layer is covered with a bilayer. For that, a force-distance curve is recorded by monitoring the tip-sample interaction via the vertical deflection of the cantilever versus its Z movement. The bilayer is not deformed without any physical contact between sample and cantilever tip (Fig. 4a). Once the tip comes into contact with the sample, the bilayer is elastically deformed up to a certain force (Fig. 4b). When the force is further increases, the membrane is plastically deformed, and the cantilever penetrates the bilayer (Fig. 4c). The force needed to pierce the bilayer is called the breakthrough force and is reflected by a jump in the vertical deflection during a force distance curve.
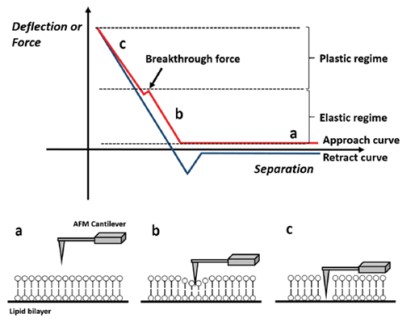
Figure 4. Schematic view of the lipid bilayer study by force distance spectroscopy
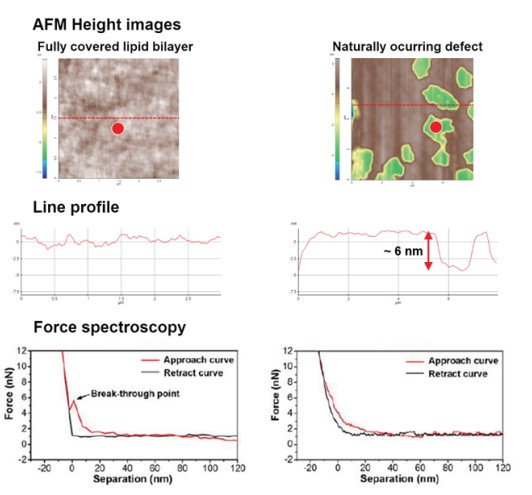
Figure 5. Force distance spectroscopy result of both lipid bilayer region and non-lipid bilayer region (red points).
In fully covered lipid bilayer, relatively flat line profile and breakthrough force was confirmed and in non lipid bilayer region, there was no breakthrough force.
Conclusion
In this app note, we confirmed vesicles and lipid bilayer formation on a solid SiO2 substrate. The surface of vesicles and lipid bilayers was imaged using Non-contact, revealing a larger surface roughness even on uniform bilayers compared to the uncovered SiO2 substrate. Moreover, we applied contact mode to mechanically scrape the bilayer and thus create a defined hole that allowed to confirm the expected bilayer thickness. Furthermore, we studied the mechanical properties of lipid bilayers using the force distance spectroscopy mode and demonstrated the occurrence of the breakthrough force as a reliable indicator for bilayer formation.
Research always starts with the most basics. And the lipid bilayer is the most basic component of the cell that is the source of life. From the study of the lipid bilayer, we may find inspiration for how cells are protected, communicated with, external or internal, and furthermore, cell differentiation and proliferation. AFM, which can measure the surface of a material in nanometers, is a very versatile technique for lipid bilayer research. In addition, since physical and mechanical information as well as image of the surface can be measured together, it will be possible to study the lipid bilayer from various perspectives.




