True Non-Contact™ Mode
True Non-Contact™ Mode Preserves Sharp Tip
AFM tips are so brittle that touching a sample will instantly reduce the resolution and quality of the image they produce. For soft and delicate samples, the tip will also damage the sample and result in inaccurate sample height measurements, something that can cost you valuable time and money. True Non-Contact™ Mode, a scan mode unique to Park AFMs, consistently produces high resolution and accurate data while maintaining the integrity of the sample.
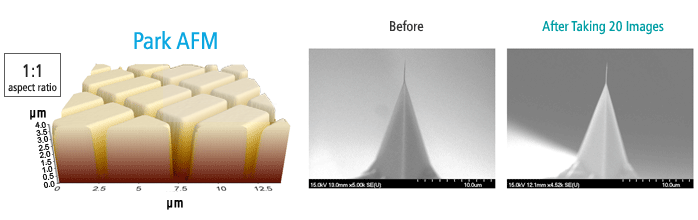
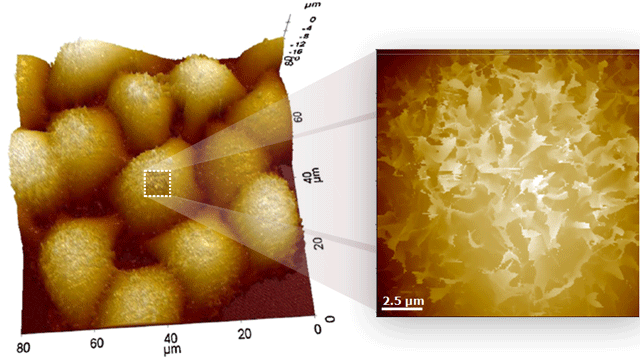
The sample image, shown in 1:1 aspect ratio, is an unprocessed raw data image of shallow trench isolation, taken by Park AFM; the depth is 3.7 μm, further confirmed by a scanning electron microscope (SEM). The two images on the right show no tip wear after taking 20 images of the sample.
Accurate Feedback by Faster Z-servo Enables True Non-Contact AFM
The benefits and the superiority of non-contact mode atomic force microscopy is well established: no tip wear, no sample damage, maintenance of high resolution imaging, and high accuracy of AFM measurement. However, only Park has achieved what True Non-Contact™ mode with its flexure-based high force Z-scanner. In True Non-Contact™ mode, the tip-sample distance is successfully maintained at a few nanometers in the net attractive regime of inter-atomic force. The small amplitude of tip oscillation minimizes the tip-sample interaction, resulting in superb tip preservation and negligible sample modification.
True Non-Contact™ Mode
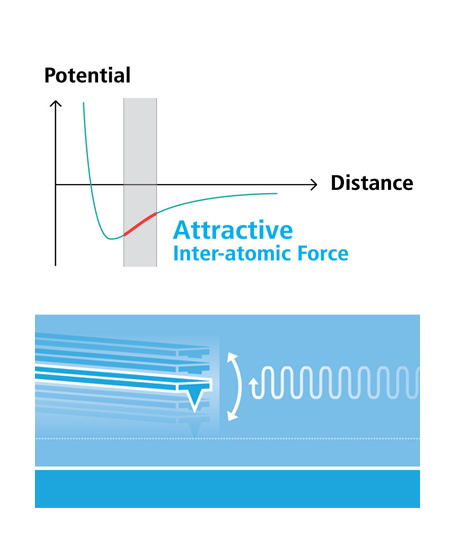
- Less tip wear = Prolonged high-resolution scan
- Non-destructive tip-sample interaction = Minimized sample modification
- Immunity from parameter dependent results

Tapping Imaging
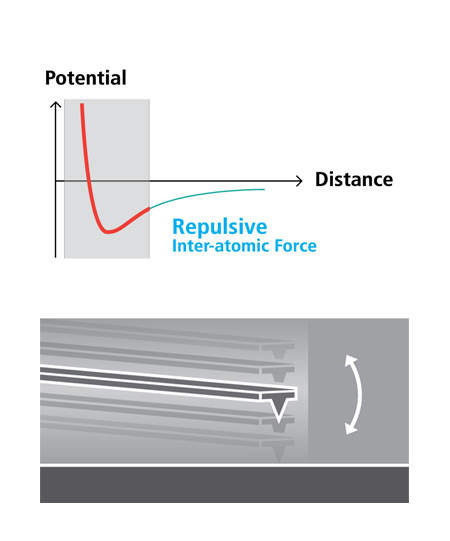
- Quick tip wear = Blurred low-resolution scan
- Destructive tip-sample interaction = Sample damage and modification
- Highly parameter-dependent

Industry Leading Z-Servo Speed
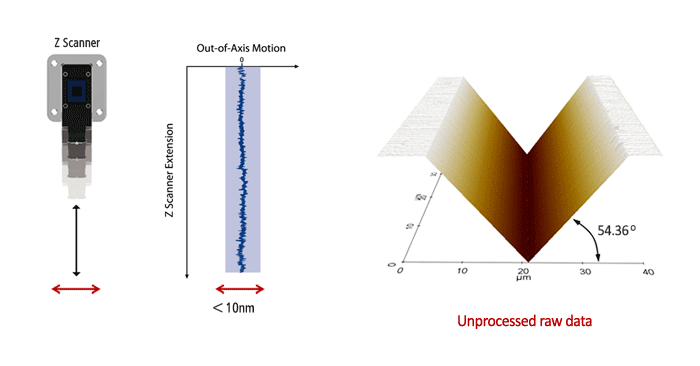
Park AFM has the fastest Z servo speed in the industry. The forward and backward scan gap is kept less than 0.15% of the scan range even during high-speed scanning.
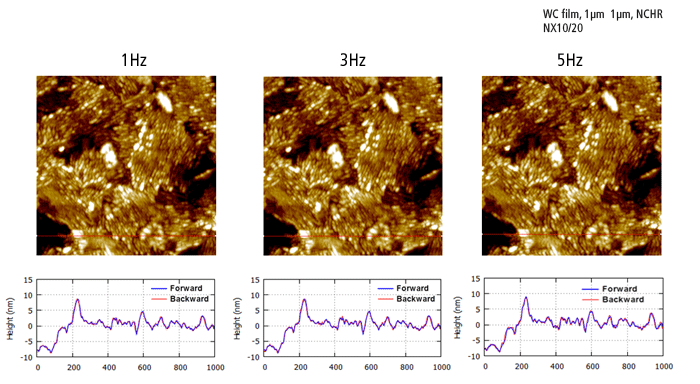
The hard, abrasive, and delicate features of the WC film sample demonstrate the advantage of the fast Z-servo speed by Park AFM. The sharp tip end is preserved during the True Non-Contact™ scan while trace and retrace are perfectly matched even at faster scan rates.
Park AFM employs industry leading fast Z-servo speed:
Park's high speed Z-servo with minimal Z scan mass and fast DSP servo control keeps the probe within the narrow range of the attractive force enabling on-contact mode AFM in ambient atmosphere.
- Z-scanner bandwidth of more than 9 kHz, or Z-servo speed of more than 62 mm/sec tip velocity
- Optimized Z servo speed with minimal Z scan mass (PSPD and tip)
- Fast DSP servo control without time delay
Crosstalk Elimination
Flat Orthogonal XY Scanning Without Scanner Bow
Park's Crosstalk Elimination removes scanner bow, allowing flat orthogonal XY scanning regardless of scan location, scan rate, and scan size. It shows no background curvature even on flattest samples, such as an optical flat, and with various scan offsets. This provides a very accurate height measurement and precision nanometrology for the most challenging problems in research and engineering.
Decoupled XY and Z Scanners
The fundamental difference between Park and its closest competitors is in the scanner architecture. Park’s unique flexure based independent XY scanner and Z scanner design allows unmatched data accuracy in nano resolution in the industry.
Accurate Surface Measurement
“Flat” sample surfaces
- Low residual bow
- No need for software processing (raw data)
- Results less dependent on scan location

Dual Servo System for Orthogonal XY Scan
The XY flexure scanner decouples the X and Y scan motion so that coupling between X and Y movement is minimized at any scan location, rate, and size. Dual position sensors provide linear and orthogonal feedback control for the highest accuracy and precision for any scan size and sample, large or small.
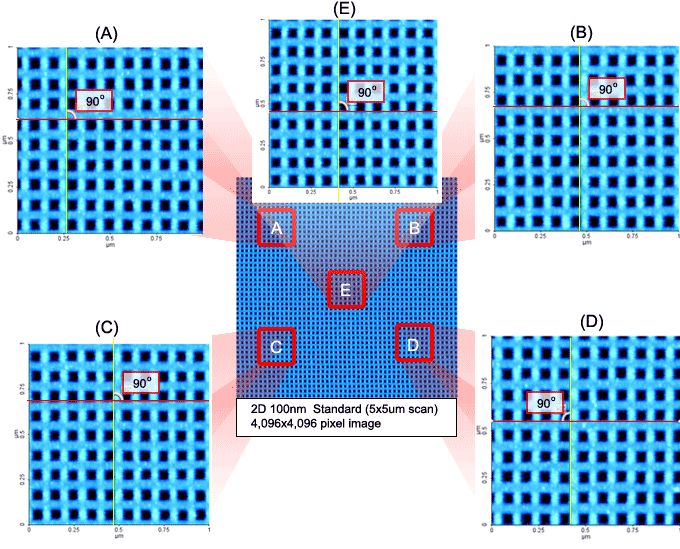 Highly Linear and Orthogonal XY Scan
Highly Linear and Orthogonal XY ScanIndustry Leading Z Scan Linearity
With Park's Crosstalk Elimination, the flexure-guided Z scanner is decoupled from the X and Y scan motion. This allows the Z scan motion to be kept in a precise straight line. The Z scan linearity of the flexure guided scanner is less than 0.015%, enabling an accurate and precise angle measurement in the nanoscale.
Park AFM provides the most accurate nano imaging data with its Crosstalk Elimination technology. It eliminates surface curvature for a wide variety of sample type and size, and it provides a flat, highly linear and orthogonal XY scan, with an accurate and precise angle measurement.
True Sample Topography
Accurate Sample Topography Measured by Low Noise Z Detector
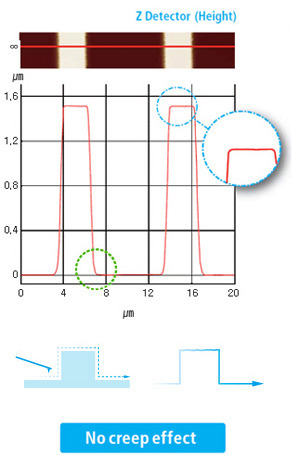
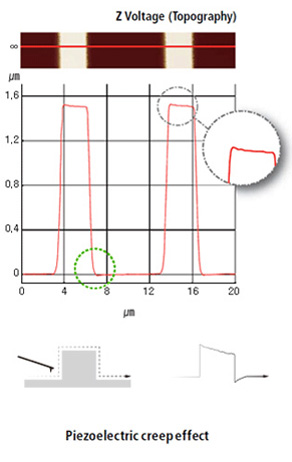
True Sample Topography™ without piezo creep error
Our AFMs are equipped with the most effective low noise Z detectors in the field, with a noise of .02 nm over large bandwidth. This produces highly accurate sample topography, no edge overshoot and no need for calibration. Just one of the many ways Park AFMs saves your time and give you better data.
- Low noise Z detector signal is used for Topography
- Low Z detector noise of 0.02 nm over large bandwidth
- No edge overshoot at the leading and trailing edges
- Calibration needs to be done only once at the factory
Park NX AFM
Conventional AFM
Park SICM
Park Scanning Ion Conductance Microscopy
In Scanning Ion Conductance Microscopy developed by Park Systems (Park SICM), a glass nanopipette (a pipette in the nanoscale) filled with an electrolyte, acts as an ion sensor that provides feedback on its location relative to a sample completely immersed in a liquid. The pipette-tip maintains its distance from the sample by keeping the ionic current constant. In comparison, Atomic Force Microscopy (AFM) typically relies on interaction of forces between a probe tip and the sample.
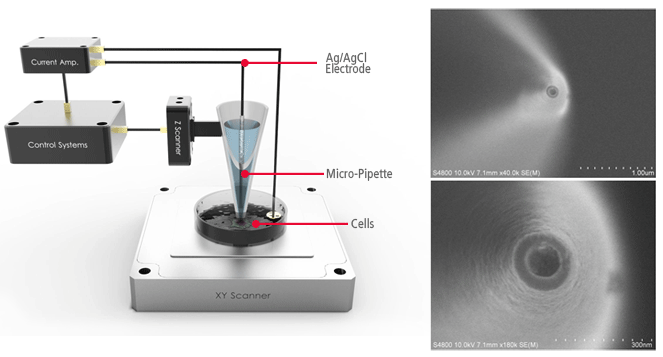
AFMs use a micro-thin cantilever and tip as a probe. For Park SICM, the pipette is a probe with an inner diameter ranging from 80 to 100 nanometers for pipettes made of glass and 30-50 nanometers for those made of quartz.
No Force, Non-Contact Imaging in Liquid
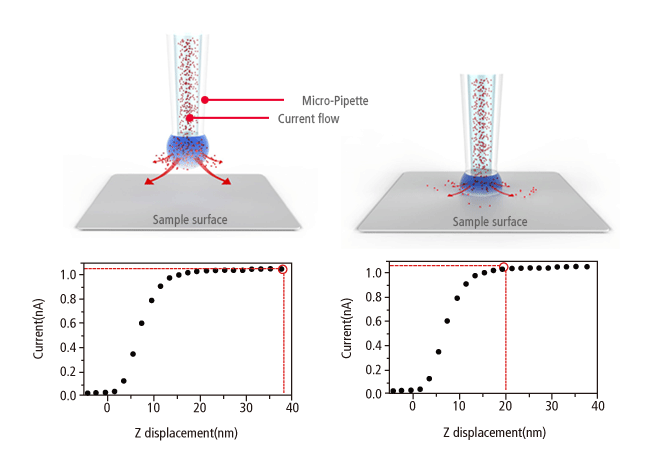
Similar to Scanning Tunneling Microscopy (STM) operating in ambient air, the Park SICM operates in a liquid without making physical contact with the sample. Electrodes on either side of the sample and pipette produce an ionic current that flows through the surrounding solution. A sensor measures the current flow, which decreases as the distance between the pipette and sample becomes smaller. This monitors the distance and directs the positioning of pipette and sample to maintain non-contact status.
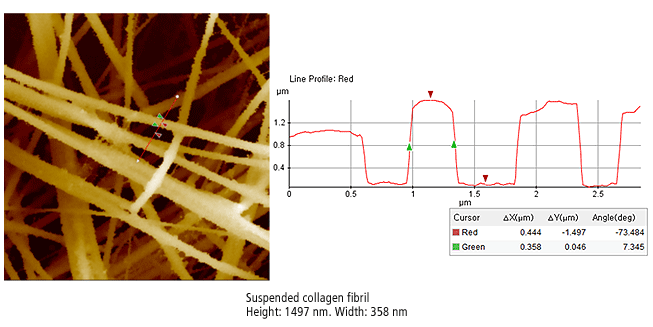
The Park SICM image on the right is suspended collagen fibril. The suspended multi-fibrils have a very complex structure, floating a several levels just microns from the bottom substrate in the liquid. This is an impossible configuration for traditional Atomic Force Microscopy as penetration into the liquid or the force of a physical probe displaces the suspended fibrils.
Live Cell Membrane Imaging with Park SICM
The cell membrane separates the interior of cells from the outside environment. Observing the functioning of living cell membranes is an increasingly important element of cell research, but it is very difficult to monitor a live cell membrane at the nanometer scale. The transparency of the membrane makes it nearly impossible to observe with optical microscopy and the membrane is too fragile to endure the force involved with AFM or Scanning Electron Microscope probes.
Park SICM, in contrast, applies no force to the sample surface and is ideal for nanoscale imaging of soft live cell membranes. The Park SICM image on the left shows live Hela cells. The cells remained live and stable throughout the duration of Park SICM imaging, showing no signs of physical deterioration. The structural complexity of live cell membranes like this is almost impossible to observe with optical microscopy or AFM