Lisa Ditscherlein,
Scientific Researcher (PhD) at MVTAT,
TU Bergakademie Freiberg,
Mechanical Process Engineering and Material Processing,
Specialization Particle Technology
NANOBUBBLES AND THEIR EFFECT ON AFM MEASUREMENTS AND ENGINEERING PROCESSES
Since the invention of devices like the surface force apparatus in 1969[1]and the atomic force microscope(AFM) in 1986[2], surface forces have been studied extensively by many scientists[3] as properties of the interfacial region are of great interest for a variety of engineering processes. Interactions between two surfaces are often quantified by classical DLVO-theory, but this theory fails if two hydrophobic surfaces approach each other in an aqueous solution. Other non-DLVO forces come into play that are of different nature and enhance attractive interactions[4]. The origin of such “hydrophobic forces” is still unknown and it is still not clear if it should be considered as a solvation force or some kind of long-ranged electrostatic or van der Waals interaction. Currently “hydrophobic forces” are considered to consist of two parts: a shortranged true hydrophobic and a long-ranged force,induced by capillary interactions. In 1994, Parker et al. were the first who proclaimed that small bridging bubbles are responsible for the long-ranged, large interaction between hydrophobic surfaces [5]. They observed steps in force-distance curves and interpreted them as coalescing nanobubbles.Some years later, in 2000, Lou et al. [6] and Ishida et al. [7] developed independently a reproducible method to generate nanobubbles on surfaces. Since then, many investigations were done on nanobubble generation[8], stability[9], size and geometry [10-13], detection methods[14], sample roughness[15] and also their impact on engineering processes and other applications.
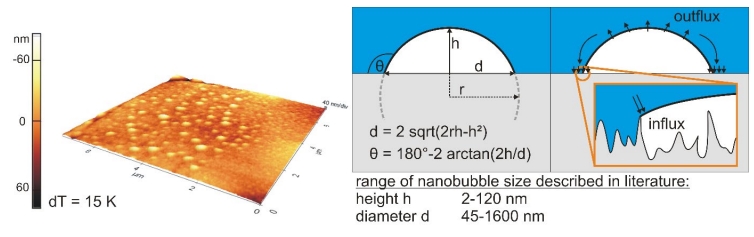
Figure 1: 3D topographical scan (isotropic axes) of a hydrophobic rough surface covered with small cap-shaped nanobubbles generated via gas oversaturation (left), geometrical parameters (middle) and scheme of stability theory (right)
In most publications, nanobubbles are generated by the so-called solvent exchange method: Here, a liquid (in most cases ethanol) with high gas solubility is replaced by a liquid with lower solubility. This leads to a local gas oversaturation and the nucleation of the bubbles [16]. Another technique is based on the same principle of gas oversaturation: sample or liquid are heated and a local temperature gradient on the surface leads to the creation of the bubbles [17]. The existence of nanobubbles has been debated for a long time, because they should dissolve in microseconds [18] but were stable for hours and even days [19]. Brenner and Lohse[20] introduced an interesting model,where the stability of the bubbles is ensured by a dynamic equilibrium between flux of gas in and out of the nanobubble,which has been further improved by Yasui et al. [21]. A dense gas layer on the surface provides enough gas for the influx in the bubble. Today it is believed that the long-time stability of nanobubbles is caused by a combination of supersaturation and contact line pinning [22, 23] which accompanies the dynamic equilibrium theory. What has been seen by Zhang and co-workers is a shrinkage of the nanobubble contact angle instead of lateral size[24].The bubble stays pinned on the three phase contact line, the Laplace pressure diverges to zero and no large inner pressure or concentration gradient between nanobubble and surrounding environment occurs. Pinning takes place due to chemical and morphological inhomogenities, even at very smooth and clean surfaces.
With phase contrast imaging it is possible to distinguish between nanobubbles and contaminations.
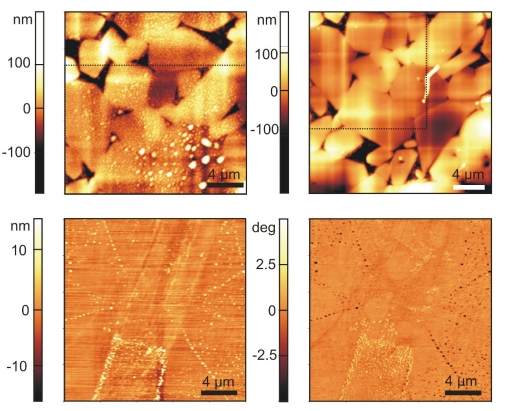
Figure 2: 2D topographical scan (top) of a rough hydrophobic surface in water with nanobubbles (left) and after drying (right) and 2D topographical scan and phase contrast image of a hydrophobic smooth surface.
In the past years, most nanobubble investigations were done using atomic force microscopy because it is a powerful tool to analyse microscopic surfaces. In principle, one can use AFM to scan the surface for bubbles directly via different scanning modes[25, 26] or indirectly via force spectroscopy[27]. Contact mode is unsuitable due to the lateral force applied to the nanobubbles which lets them coalesce or even detach from the surface. This mode has only been used to prove the soft character of the cap-shaped domes [27]. Commonly, intermittent/Tapping® mode is applied to detect the bubbles since lateral forces are minimized as a consequence of cantilever oscillation. Amplitude ratios (set point amplitude to free amplitude) should lie above 0.7 as otherwise deformation of the bubbles becomes strong [28, 29] and distorts the result. Often, phase contrast imaging is done simultaneously to facilitate the distinction between bubble and surface. A more seldom-used method is frequency modulation/true non-contact mode. Here, the cantilever oscillates at its resonant frequency close to the nanobubble-covered sample with a small amplitude without touching it. With this mode, deformation of nanobubbles is minimized. In recent years, the PeakForce® mode has been used for nanobubble detection as well. This mode works at frequencies below resonance and tracks the maximum force load on the tip in real time—which is advantageous compared to tapping mode, where it is difficult to quantify this force. An influencing factor is the chosen imaging force: the larger the force, the higher the deformation of the bubbles. In order to detect nanobubbles it is obvious that a soft and sharp cantilever is needed due to the soft matter character of the bubbles. Normally, cantilevers with spring constants between 0.2 and 4.8 N/m are used. It is to say that, besides AFM mode, the cantilever tip influences the scanning results [30]so that a recalculation of the geometrical size is necessary, if possible. Although the AFM provides three-dimensional information there are some drawbacks: a perturbation of the nanobubbles by the tip is inevitable. Another problem is the inability of the AFM to distinguish between gaseous domains and contamination: it is important to work under clean conditions, e.g. using only glass syringes and clean cantilevers [31].Therefore, alternative observation techniques are utilized like rapid cryofixation[32], infrared spectroscopy to determine the presence of nanobubbles[33], interference-enhanced reflection microscopy [34] to study bubble growth, attenuated total internal reflection microscopy in combination with other spectroscopic methods that investigate the formation of nanobubbles [35], or confocal microscopy to name but a few.
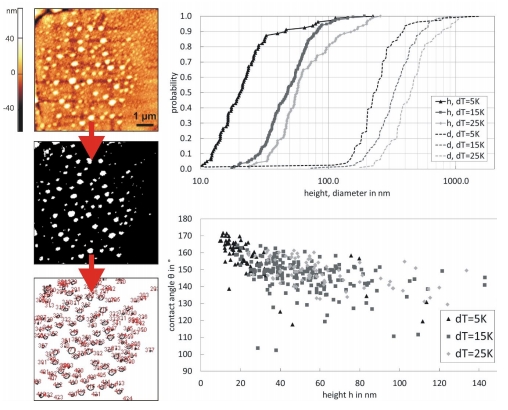
Figure 3: Evaluation of nanobubbles on a rough hydrophobic surface via ImageJ by binarisation (a certain threshold has to be chosen), geometrical size distributions of nanobubbles and dependency of contact angle on height for different temperature gradients
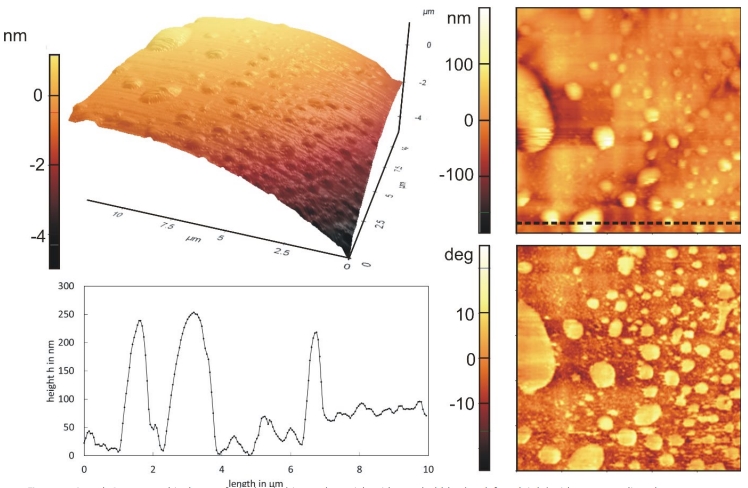
Figure 4: 3D and 2D topographical scan of a hydrophobic rough particle with nanobubbles (top left and right) with corresponding phase contrast image (bottom right) and line profile (bottom left).
Interestingly, nearly all examined surfaces like silanized Si-wafers, mica, HOPG or PS are relatively smooth with rms 100 nm) and often inhomogeneous surfaces like crushed particles, sintered or abraded surfaces. Measurement on rough surfaces is more complicated, since peaks and valleys influence results if parameters are improperly set and it is often more time-consuming. For such surfaces there is a need of statistically valid results because of the highly variable interaction area that changes from point to point. This can only be accomplished by a comprehensive sampling and a high number of measurements. Consequently, for force distance spectroscopy hundreds or thousands of curves and an evaluation routine becomes necessary. Previous works on rough surfaces show the impact of wettability and roughness on nanobubble count, stability and therefore adhesive interactions [36-38]: whilst the probability of capillary interactions is low on hydrophilic surfaces (θ = 80°), it is more than doubled on hydrophobic samples (θ = 104°). This leads to much stronger adhesion between the two surfaces. Contrary to results for hydrophilic systems, adhesion is increased with higher roughness. The explanation for this behaviour is a larger amount of bubbles nested in a stable position inside the pores and asperities. On the other hand, access for the second interacting surface to the nanobubble has to be ensured as otherwise no capillary bridge is formed. Another, very interesting aspect seen on slightly rough, but also on technical rough surfaces, is the increased stability of nanobubbles. Only small contact forces (below 5 nN) are necessary to move bubbles on smooth surfaces. In contrast, on rough surfaces they are only deformed but keep pinned to the asperities and pores. Contact forces tenfold stronger are needed to move them.
Engineering processes like flotation, agglomeration or filtration benefit from the microscopic findings about nanobubbles and can be optimized. For mineral flotation, an engineering process that separates hydrophobic from hydrophilic materials, the attachment between bubble and valuable material is of great importance. Normally, good flotation performance is limited to particle sizes between 10 and 100 µm (minerals) or 50 to 600 µm (coal). Nanobubbles can increase the recovery of finer particles by improving film rupture [39, 40] and have been studied for coal, sheelite, chalcopyrite and quartz. Less frother is necessary because they stabilize the froth and the necessary amount of air is reduced [41]. A large quantity of submicron bubbles can be achieved by ultrasound or hydrodynamic cavitation [42]. Progress is made in the field of nanobubble-nanoparticle contact. Contrary to froth flotation, particle-bubble contact is not achieved by collision but by new nucleation of nanobubbles directly onto the nanoparticles [43]. Agglomeration, another engineering process, is also strongly influenced by the presence of the bubbles. Nanobubbles promote agglomeration between hydrophobic particles due to capillary interactions between them, a phenomenon that holds true for fine as well as coarse particles [44]. In [45], an enhancement of agglomeration can be seen in agglomeration size and particle-particle interactions. Furthermore, agglomerates that contain nanobubbles are more stable at higher shear rates[46]. In deep bed filtration experiments, an improvement of filtration 10 NANOscientific www.nano-scientific.org Figure 4: 3D and 2D topographical scan of a hydrophobic rough particle with nanobubbles (top left and right) with corresponding phase contrast image (bottom right) and line profile (bottom left). www.nanoscientific.org NANOscientific 11 efficiency can be observed, too. Here, nanobubbles are sitting on poorly wetted foam filters and keep inclusion particles trapped [37]. The amount of nanobubbles determines the degree of efficiency. These results are not only interesting for conventional deep bed filtration processes but also for the purification of metal melts [47, 48].
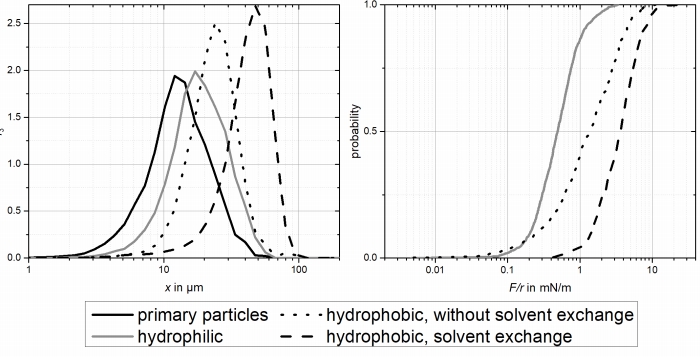
Figure 5: agglomerate size (left) and particle-particle interaction dependency on wetting properties.
The formation of nanobubbles leads to larger adhesive forces and therefore larger agglomerates
There are many more promising fields for the application of nanobubbles like wastewater treatment [49], micro- and nanofluidics[50] or electrolysis [51]. The research topic “nanobubbles” provides interesting opportunities in the next few years. It remains exciting.
[1] D. Tabor and R. H. S. Winterton, "The direct measurement of normal and retarded van der Waals forces," Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences, vol. 312, pp. 435-450, 1969.
[2] G. Binnig, C. F. Quate, and C. Gerber, "Atomic force microscope," Physical Review Letters, vol. 56, pp. 930-933, 1986.
[3] J. N. Israelachvili, Intermolecular and Surface Forces, 3 ed. Oxford: Elsevier LTD., 2011.
[4] H. K. Christenson and P. M. Claesson, "Direct measurements of the force between hydrophobic surfaces in water," Advances in Colloid and Interface Science, vol. 91, pp. 391-436, 2001.
[5] J. L. Parker, P. M. Claesson, and P. Attard, "Bubbles, cavities, and the long-ranged attraction between hydrophobic surfaces," Journal of Physical Chemistry®, vol. 98, pp. 8468-8480, 1994.
[6] S. T. Lou, Z. Q. Ouyang, Y. Zhang, X. J. Li, J. Hu, M. Q. Li, et al., "Nanobubbles on solid surface imaged by atomic force microscopy," Journal of Vacuum Science and Technology B: Microelectronics and Nanometer Structures, vol. 18, pp. 2573-2575, 2000.
[7] N. Ishida, T. Inoue, M. Miyahara, and K. Higashitani, "Nano bubbles on a hydrophobic surface in water observed by tapping-mode atomic force microscopy," Langmuir, vol. 16, pp. 6377-6380, 2000.
[8] M. A. J. Van Limbeek and J. R. T. Seddon, "Surface nanobubbles as a function of gas type," Langmuir, vol. 27, pp. 8694-8699, 2011.
[9] Y. Sun, G. Xie, Y. Peng, W. Xia, and J. Sha, "Stability theories of nanobubbles at solid-liquid interface: A review," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 495, pp. 176-186, 2016.
[10] R. P. Berkelaar, J. R. T. Seddon, H. J. W. Zandvliet, and D. Lohse, "Temperature dependence of surface nanobubbles," ChemPhysChem, vol. 13, pp. 2213-2217, 2012.
[11] B. Bhushan, Y. Pan, and S. Daniels, "AFM characterization of nanobubble formation and slip condition in oxygenated and electrokinetically altered fluids," Journal of Colloid and Interface Science, vol. 392, pp. 105-116, 2013.
[12] B. M. Borkent, S. De Beer, F. Mugele, and D. Lohse, "On the shape of surface nanobubbles," Langmuir, vol. 26, pp. 260-268, 2010.
[13] D. Li and X. Zhao, "Micro and nano bubbles on polystyrene film/ water interface," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 459, pp. 128-135, 2014.
[14] D. Lohse and X. Zhang, "Surface nanobubbles and nanodroplets," Reviews of Modern Physics, vol. 87, 2015.
[15] J. Yang, J. Duan, D. Fornasiero, and J. Ralston, "Very small bubble formation at the solid-water interface," Journal of Physical Chemistry B, vol. 107, pp. 6139-6147, 2003.
[16] M. A. Hampton, B. C. Donose, and A. V. Nguyen, "Effect of alcohol-water exchange and surface scanning on nanobubbles and the attraction between hydrophobic surfaces," Journal of Colloid and Interface Science, vol. 325, pp. 267-274, 2008.
[17] C. Xu, S. Peng, G. G. Qiao, V. Gutowski, D. Lohse, and X. Zhang, "Nanobubble formation on a warmer substrate," Soft Matter, vol. 10, pp. 7857-7864, 2014.
[18] S. Ljunggren and J. C. Eriksson, "The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 129-130, pp. 151-155, 1997.
[19] X. H. Zhang, A. Quinn, and W. A. Ducker, "Nanobubbles at the interface between water and a hydrophobic solid," Langmuir, vol. 24, pp. 4756-4764, 2008.
[20] M. P. Brenner and D. Lohse, "Dynamic equilibrium mechanism for surface nanobubble stabilization," Physical Review Letters, vol. 101, 2008.
[21] K. Yasui, T. Tuziuti, W. Kanematsu, and K. Kato, "Advanced dynamic-equilibrium model for a nanobubble and a micropancake on a hydrophobic or hydrophilic surface," Physical Review E - Statistical, Nonlinear, and Soft Matter Physics, vol. 91, 2015.
[22] Y. Liu and X. Zhang, "A unified mechanism for the stability of surface nanobubbles: Contact line pinning and supersaturation," Journal of Chemical Physics, vol. 141, 2014.
[23] D. Lohse and X. Zhang, "Pinning and gas oversaturation imply stable single surface nanobubbles," Physical Review E, vol. 91, p. 031003, 03/27/ 2015.
[24] X. Zhang, D. Y. C. Chan, D. Wang, and N. Maeda, "Stability of interfacial nanobubbles," Langmuir, vol. 29, pp. 1017-1023, 2013.
[25] W. Walczyk, P. M. Schön, and H. Schönherr, "The effect of PeakForce tapping mode AFM imaging on the apparent shape of surface nanobubbles," Journal of Physics Condensed Matter, vol. 25, 2013.
[26] C. W. Yang, Y. H. Lu, and I. S. Hwang, "Imaging surface nanobubbles at graphite-water interfaces with different atomic force microscopy modes," Journal of Physics Condensed Matter, vol. 25, 2013.
[27] L. Ditscherlein, J. Fritzsche, and U. A. Peuker, "Study of nanobubbles on hydrophilic and hydrophobic alumina surfaces," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 497, pp. 242-250, 2016.
[28] X. H. Zhang, N. Maeda, and V. S. J. Craig, "Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions," Langmuir, vol. 22, pp. 5025-5035, 2006.
[29] S. Yang, E. S. Kooij, B. Poelsema, D. Lohse, and H. J. W. Zandvliet, "Correlation between geometry and nanobubble distribution on HOPG surface," EPL, vol. 81, 2008.
[30] W. Walczyk and H. Schönherr, "Characterization of the interaction between AFM tips and surface nanobubbles," Langmuir, vol. 30, pp. 7112-7126, 2014.
[31] R. P. Berkelaar, E. Dietrich, G. A. M. Kip, E. S. Kooij, H. J. W. Zandvliet, and D. Lohse, "Exposing nanobubble-like objects to a degassed environment," Soft Matter, vol. 10, pp. 4947-4955, 2014.
[32] M. Switkes and J. W. Ruberti, "Rapid cryofixation/freeze fracture for the study of nanobubbles at solid-liquid interfaces," Applied Physics Letters, vol. 84, pp. 4759-4761, 2004.
[33] X. H. Zhang, A. Khan, and W. A. Ducker, "A nanoscale gas state," Physical Review Letters, vol. 98, 2007.
[34] S. Karpitschka, E. Dietrich, J. R. T. Seddon, H. J. W. Zandvliet, D. Lohse, and H. Riegler, "Nonintrusive optical visualization of surface nanobubbles," Physical Review Letters, vol. 109, 2012.
[35] C. U. Chan and C. D. Ohl, "Total-internal-reflection-fluorescence microscopy for the study of nanobubble dynamics," Physical Review Letters, vol. 109, 2012.
[36] J. Fritzsche and U. A. Peuker, "Wetting and Adhesive Forces on Rough Surfaces – An Experimental and Theoretical Study," Procedia Engineering, vol. 102, pp. 45-53, 2015.
[37] F. Heuzeroth, J. Fritzsche, and U. A. Peuker, "Wetting and its influence on the filtration ability of ceramic foam filters," Particuology, vol. 18, pp. 50-57, 2015.
[38] L. Ditscherlein, A. Schmidt, E. Storti, C. G. Aneziris, and U. A. Peuker, "Impact of the Roughness of Alumina and Al2O3-C Substrates on the Adhesion Mechanisms in a Model System," Advanced Engineering Materials, 2017.
[39] K. W. Stöckelhuber, "Stability and rupture of aqueous wetting films," European Physical Journal E, vol. 12, pp. 431-435, 2003.
[40] S. Calgaroto, K. Q. Wilberg, and J. Rubio, "On the nanobubbles interfacial properties and future applications in flotation," Minerals Engineering, vol. 60, pp. 33-40, 2014.
[41] A. Sobhy and D. Tao, "Nanobubble column flotation of fine coal particles and associated fundamentals," International Journal of Mineral Processing, vol. 124, pp. 109-116, 2013.
[42] R. Etchepare, H. Oliveira, M. Nicknig, A. Azevedo, and J. Rubio, "Nanobubbles: Generation using a multiphase pump, properties and features in flotation," Minerals Engineering, vol. 112, pp. 19-26, 2017. [43] M. Zhang and J. R. T. Seddon, "Nanobubble–Nanoparticle
Interactions in Bulk Solutions," Langmuir, vol. 32, pp. 11280-11286, 2016/11/01 2016.
[44] M. Cournil, F. Gruy, P. Gardin, and H. Saint-Raymond, "Modelling of solid particle aggregation dynamics in non-wetting liquid medium," Chemical Engineering and Processing: Process Intensification, vol. 45, pp. 586-597, 2006.
[45] P. Knüpfer, L. Ditscherlein, and U. A. Peuker, "Nanobubble enhanced agglomeration of hydrophobic powders," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 530, pp. 117-123, 10/5/ 2017.
[46] F. Gruy, M. Cournil, and P. Cugniet, "Influence of nonwetting on the aggregation dynamics of micronic solid particles in a turbulent medium," Journal of Colloid and Interface Science, vol. 284, pp. 548-559, 2005.
[47] C. Voigt, L. Ditscherlein, E. Werzner, T. Zienert, R. Nowak, U. Peuker, et al., "Wettability of AlSi7Mg alloy on alumina, spinel, mullite and rutile and its influence on the aluminum melt filtration efficiency," Materials and Design, vol. 150, pp. 75-85, 2018.
[48] M. Cournil, F. Gruy, P. Gardin, and H. Saint-Raymond, "Experimental Study and Modeling of Inclusion Aggregation in Turbulent Flows to Improve Steel Cleanliness," physica status solidi (a), vol. 189, pp. 159- 168, 2002/01/01 2002.
[49] A. Agarwal, W. J. Ng, and Y. Liu, "Principle and applications of microbubble and nanobubble technology for water treatment," Chemosphere, vol. 84, pp. 1175-1180, 2011.
[50] Y. Wang and B. Bhushan, "Boundary slip and nanobubble study in micro/nanofluidics using atomic force microscopy," Soft Matter, vol. 6, pp. 29-66, 2009.
[51] L. Zhang, Y. Zhang, X. Zhang, Z. Li, G. Shen, M. Ye, et al., "Electrochemically controlled formation and growth of hydrogen